Beautiful Sunday morning on the empty campus. CHARLIE, LARRY,
AMITA, and ALAN huddle around a pool filled with milky water.
ALAN
This is in your lesson plan why?
CHARLIE
It's an interesting study in
transitional system analysis --
ALAN
I thought you were teaching 'Advanced
Number Theory'?
CHARLIE
Well, the Riemann Zeta Function --
(busted)
...yeah.
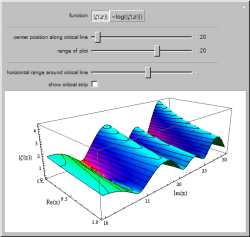
Charlie fiddles with an equation at his board as he talks to
Don.
LARRY
A scientist is never so diligent as
when he himself is the experimental
subject.
CHARLIE
I was diligent! I'm calculating
possible concentration-temperature
coefficients--
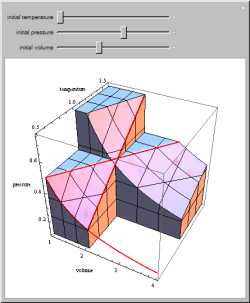
Explore the thermodynamic space P-V-T of an ideal gas and see relationships between isochors, isobars, and isotherms and
their
partial derivatives.
CHARLIE
I was about to tell Don that, unless
we have a thousand psychopaths, the
poisoner's motives and/or actions
should be detectable from the
'normal' social dynamics, whatever
those are.
FLASH CHARLIE VISION
of the CROSS SECTION of an apartment building... layers of
electrical and plumbing and cable TV line schematics laying
in...
BACK TO CHARLIE
CHARLIE (cont'd)
I could use Social and Bayesian
Network Analysis to uncover hidden
dynamics and covert architectures of
the cult.
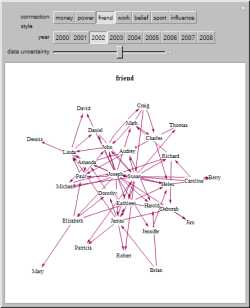
In a social network, people are points (referred to as
nodes) connected by
edges
(referred to as ties). For example, if the nodes were movie actors, such as Ed Harris and Judd Hirsch, they could be
tied together by a movie they both starred in. Within this randomly generated data, Susan and Joseph both seem to be
highly influential.
She drops the hair follicles in a bluish solution.
RIDENHOUR (cont'd)
Atomic absorption spectrometry.
She puts the solution in a machine and starts the process.
RIDENHOUR (cont'd)
This bad boy zaps it all the way from
liquid into free atoms, measuring the
concentration of the Arsenic.
The digital display on the machine reads: 0 PPB
RIDENHOUR (cont'd)
A normal person's hair would read 10
to 20 PPB -- parts per billion.
DAVID
And a murder victim?
RIDENHOUR
Above 10,000 PPB will be fatal.
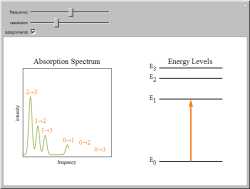
This Demonstration shows some general qualitative features of absorption spectroscopy, common to all types of
spectroscopy. Given a stack of quantized energy levels--atomic, molecular, or nuclear--radiation will be absorbed
when its frequency ν matches one of the energy level differences, according to the Bohr condition.




